Abstract
Introduction: Less than 5% of patients with MDS present with thrombocytopenia as an isolated abnormality (MDS-IT). There have been few systematic studies on MDS-IT and data regarding its course and prognosis are conflicting. Previous studies have defined MDS-IT based on the IPSS thresholds (Hb ≥10 g/dL; ANC ≥1.8×10 9/L; PLT <100×10 9/L). However, these were developed for prognostic, not diagnostic purposes which means that mild anemia and/or neutropenia might be present concomitantly with "isolated" thrombocytopenia. We aimed to investigate the characteristics, overall survival (OS), and leukemia-free survival (LFS) of patients with MDS-IT.
Methods: We identified patients who had PLT <150 ×10 9/L, Hb >13 g/dL (men) or >12 g/dL (women), and ANC ≥1.8 ×10 9/L, registered in the Hellenic National Registry of Myelodysplastic and Hypoplastic Syndromes which includes 2792 patients (analysis cut-off date; July 7, 2016). Patients were divided into 4 groups: group 1 had PLT 149-100 ×10 9/L; group 2, 99-50 ×10 9/L; group 3, <50 ×10 9/L; and group 4, <25 ×10 9/L. We also collected data from the Hellenic National ITP Registry which includes 1317 adult patients with ITP.
Results: A total of 77 patients (45 men; 32 women) with MDS-IT were identified (2.9% of total MDS cohort). Of these, 28.6% were classified in group 1; 49.4% in group 2; 14.3% in group 3; and 7.8% in group 4. Median PLT count was 87 ×10 9/L (12-139 ×10 9/L), WBC count 4.6 ×10 9/L, and Hb 13.6 g/dL. Bone marrow (BM) blasts ranged from 0-9% (median, 2%). Median follow-up was 51.0 months (41.6-60.4), during which 15 (19.5%) patients died. AML developed in 9 patients (11.7%). Histologically, MDS with multilineage dysplasia (MLD) was seen in 77.6% whereas MDS with excess blasts (EB) and MDS with single lineage dysplasia (SLD) comprised 10.7% and 11.9% of cases, respectively. Most patients (73.5%) had lower-risk MDS on the IPSS-R (i.e. IPSS-R ≤3.5). Of the 59 patients with cytogenetic data, 83.1% had favorable, 13.5% intermediate, and 3.4% poor risk cytogenetics. Most (40) had a normal karyotype followed by isolated del(20q) (6). All patients with del(20q) showed a characteristic set of clinical features: age >60 years, blasts 0-3%, bilineage (erythroid/megakaryocytic) dysplasia, and increased reticulin fibrosis. There were no significant differences between any of the 4 PLT groups regarding age, sex, IPSS-R, cytogenetics, BM blasts, and histology.
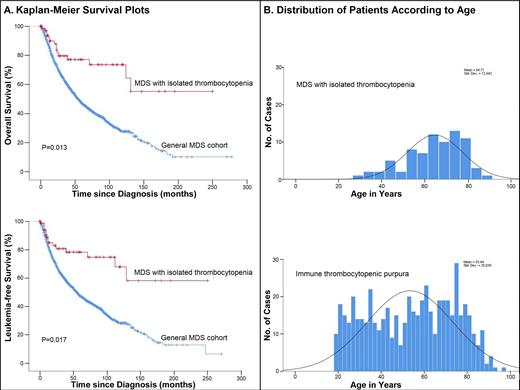
Median OS was 109 months (95% CI 103-115) and LFS 108 months (101-115). Our results showed no significant difference in OS (P=0.891) and LFS (P=0.871) between the 4 PLT groups. As compared with total MDS cohort, MDS-IT occurred at younger age (64.7 vs. 72.4 years, P<0.001). In a Kaplan-Meier analysis, patients with MDS-IT had markedly longer OS and LFS than patients in the total MDS cohort, even after adjustment for age, sex, IPSS-R, blasts, and PLT (P=0.013 for OS; P=0.017 for LFS) (Figure 1A). There were no differences in the top causes of death: infection was the commonest cause followed by disease progression and cardiovascular disease. Major bleeding comprised 10.3% of deaths in MDS-IT vs. 12.7% in total MDS cohort (P=0.217).
In comparing MDS-IT with ITP, the median age at diagnosis was 66.0 years for MDS-IT and 49.0 years for ITP (P<0.001).MDS-IT was uncommon in patients <50 or >80 years. Its incidence reached a peak between the ages of 70-79 years, whereas ITP occurred at a more constant level over time (Figure 1B). Women predominated in ITP and men in MDS-IT (P=0.007). Overall, ITP was associated with more marked thrombocytopenia than MDS-IT (15.0 ×10 9/L vs. 87.0 ×10 9/L) (P<0.001). Median WBC count was higher in ITP (7.6 ×10 9/L vs. 4.6 ×10 9/L; P<0.001). Median Hb was similar in the 2 groups. Patients with ITP had longer OS than MDS-IT (P<0.001).
Conclusions: In one of the largest reported series, we conclude that MDS-IT is associated with MDS-MLD, favorable cytogenetics, lower-risk IPSS-R, high survival rate, and a low risk of AML evolution. Our data suggest that the superior prognosis in MDS-IT than general MDS may have intrinsic genomic underpinnings as survival curves remained unchanged after correcting for age, sex, blasts and IPSS-R. Importantly, no significant differences in OS and LFS were noted between the 4 PLT subgroups, suggesting that the degree of thrombocytopenia does not correlate with mortality in MDS-IT. From the diagnostic standpoint, age <50 or >80 years and PLT <25 ×10 9/L favored a diagnosis of ITP over MDS-IT.
Viniou: Sandoz: Research Funding; Takeda: Research Funding; Novartis: Honoraria, Research Funding; Sanofi: Research Funding; Janssen: Honoraria, Research Funding; Pfizer: Research Funding; Abbvie: Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Roche: Research Funding; Astellas: Research Funding; Celgene: Research Funding. Vassilakopoulos: Dr. Reddy's: Research Funding; Amgen: Honoraria, Research Funding; GlaxoSmithKline: Honoraria, Other: Travel; AbbVie: Consultancy, Honoraria; Integris: Honoraria; Pfizer: Research Funding; Roche: Consultancy, Honoraria, Other: Travel; Takeda: Consultancy, Honoraria, Other: Travel, Research Funding; Genesis Pharma: Consultancy, Honoraria, Other: Travel; Merck: Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Karyopharm: Research Funding; AstraZeneca: Honoraria. Hatzimichael: Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Honoraria; Gilead: Honoraria; Janssen Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Genesis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Pharmathen- Innovis: Honoraria; GSK: Honoraria; Bristol Myersr Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees. Symeonidis: Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi/Genzyme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Demo: Research Funding; MSD: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; WinMedica: Research Funding; Astellas: Consultancy, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GenesisPharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal